
P [Ne] 3s2 3p3 3e —> P3+ [Ne] 3s2 3p0. Dr. S. M. Condren. Dynamic
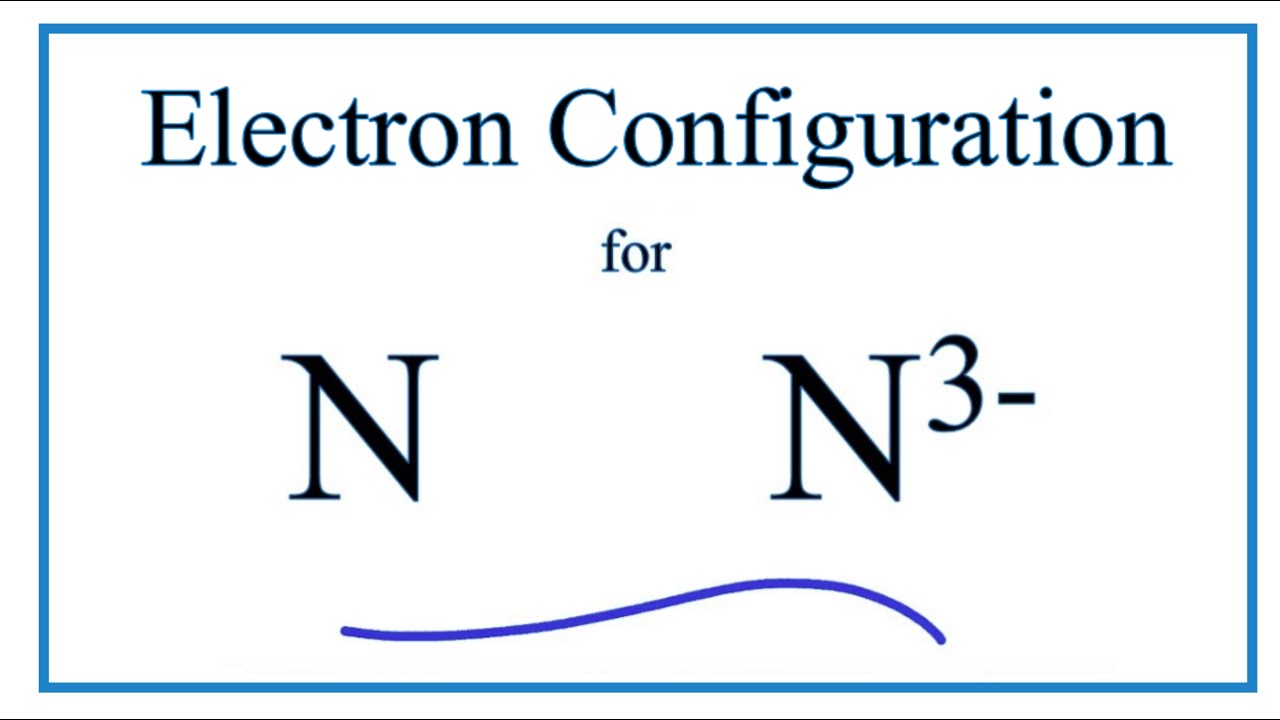
N 3- Electron Configuration (Nitride Ion) Wayne Breslyn 726K subscribers Subscribed 67K views 4 years ago In this video we will write the electron configuration for N 3-, the Nitride ion..

Manganese Electron Configuration Ground State / How Many Unpaired
By extrapolation, we expect all the group 2 elements to have an ns2 electron configuration. Exercise 6.9.1 6.9. 1. Use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. Answer: All have an ns2np5 electron configuration, one electron short of a noble gas electron configuration.
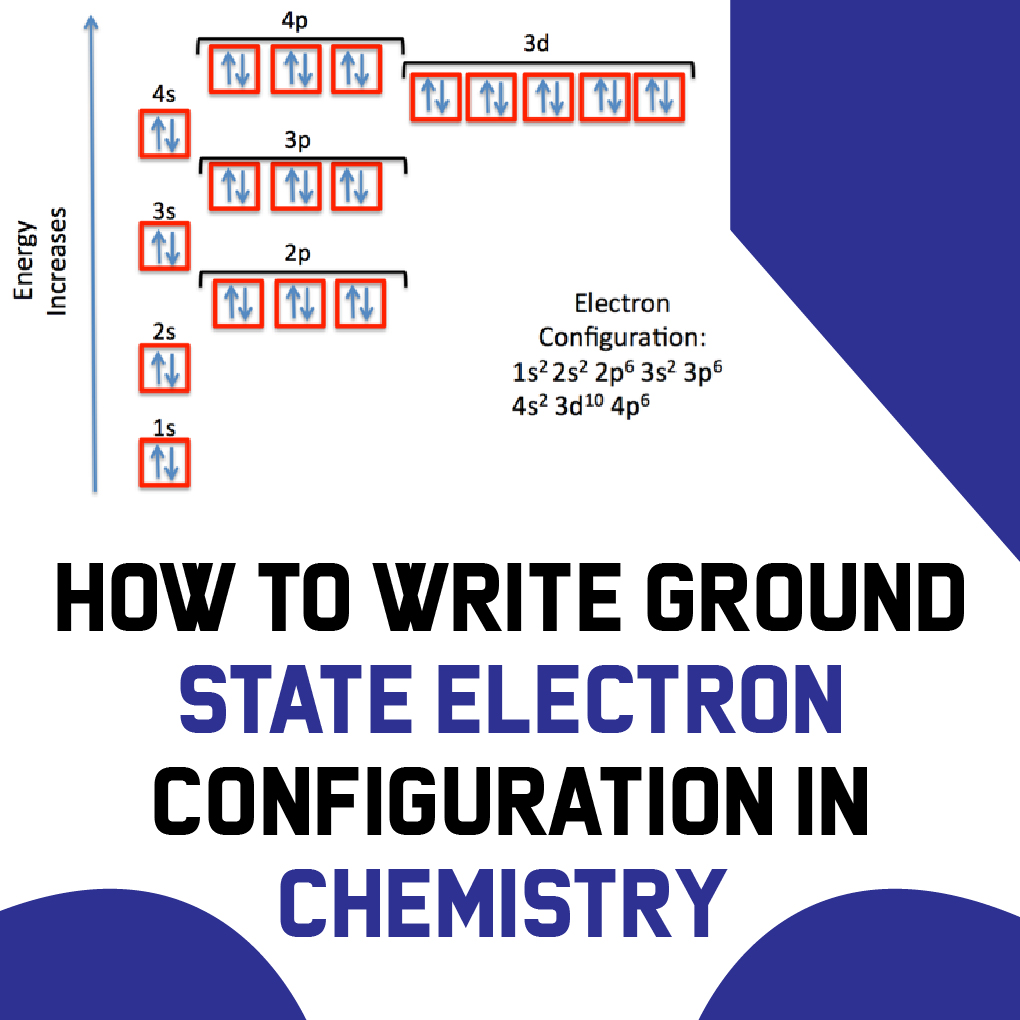
How to Write Ground State Electron Configuration in Chemistry
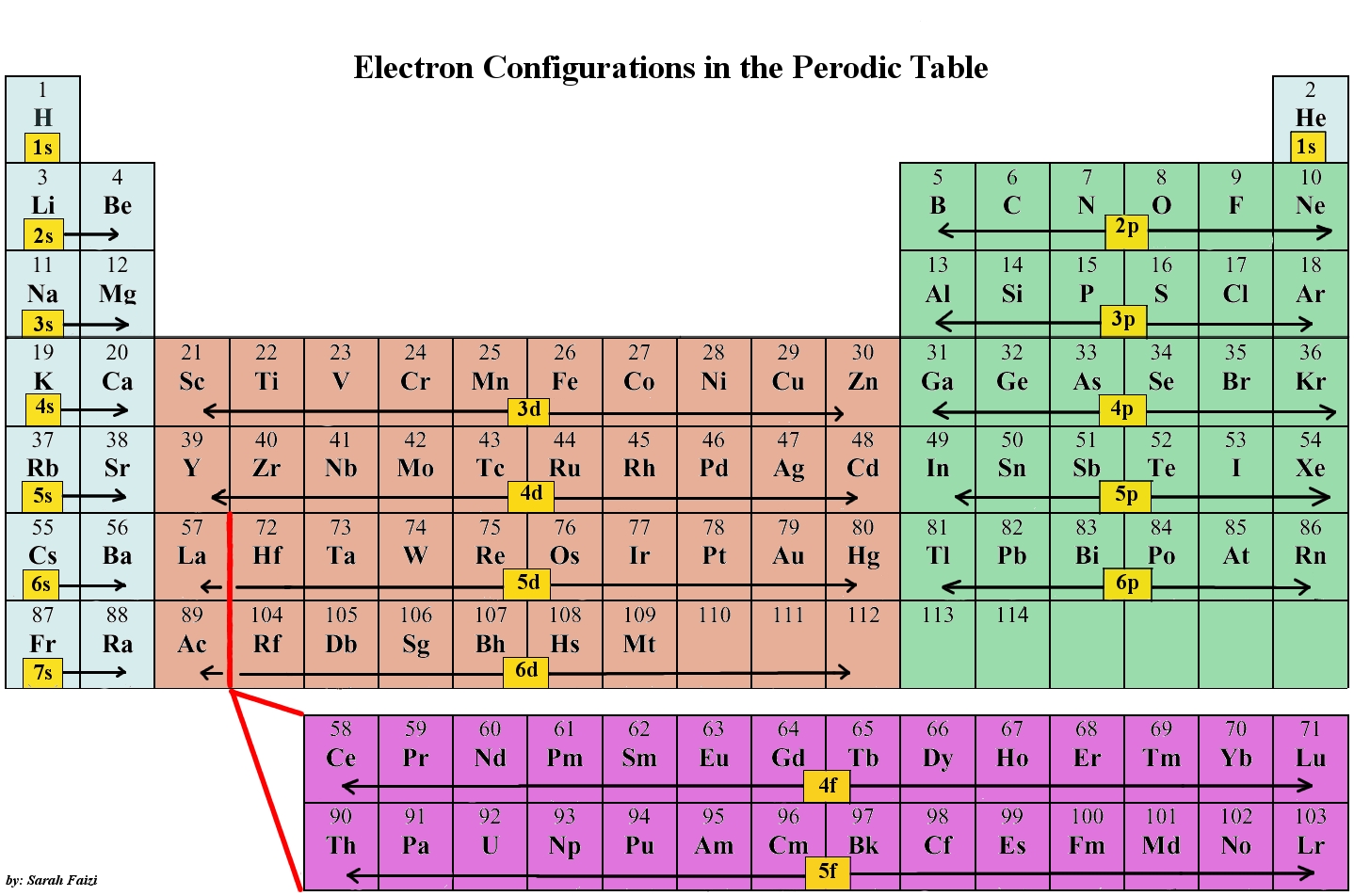
Notation Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.
Sodium Electron Configuration Diagram, Electronic Configuration The
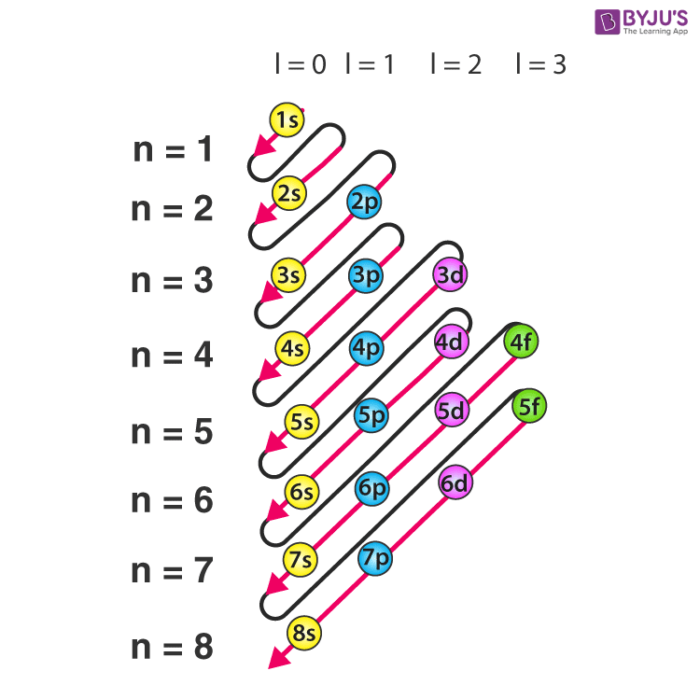
Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,.. For example, an Iodine atom has its outmost electrons in the 5p.

N 3 Electron Configuration (Nitride Ion) Electron configuration
Oct 27, 2016 1s22s22p6 Explanation: Nitrogen has an initial electron configuration of 1s22s22p3 If Nitrogen gains three electrons the 2p orbitals will have 6 electrons giving 2p6 This creates the electron configuration of Neon making the atom much more stable than the initial or ground state.
Electron Configuration Basic introduction YoutuBeRandom
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Periodic Table Electron Configuration
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
Electronic Configurations Chemwiki
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
N 3 Electron Configuration (Nitride Ion) YouTube
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
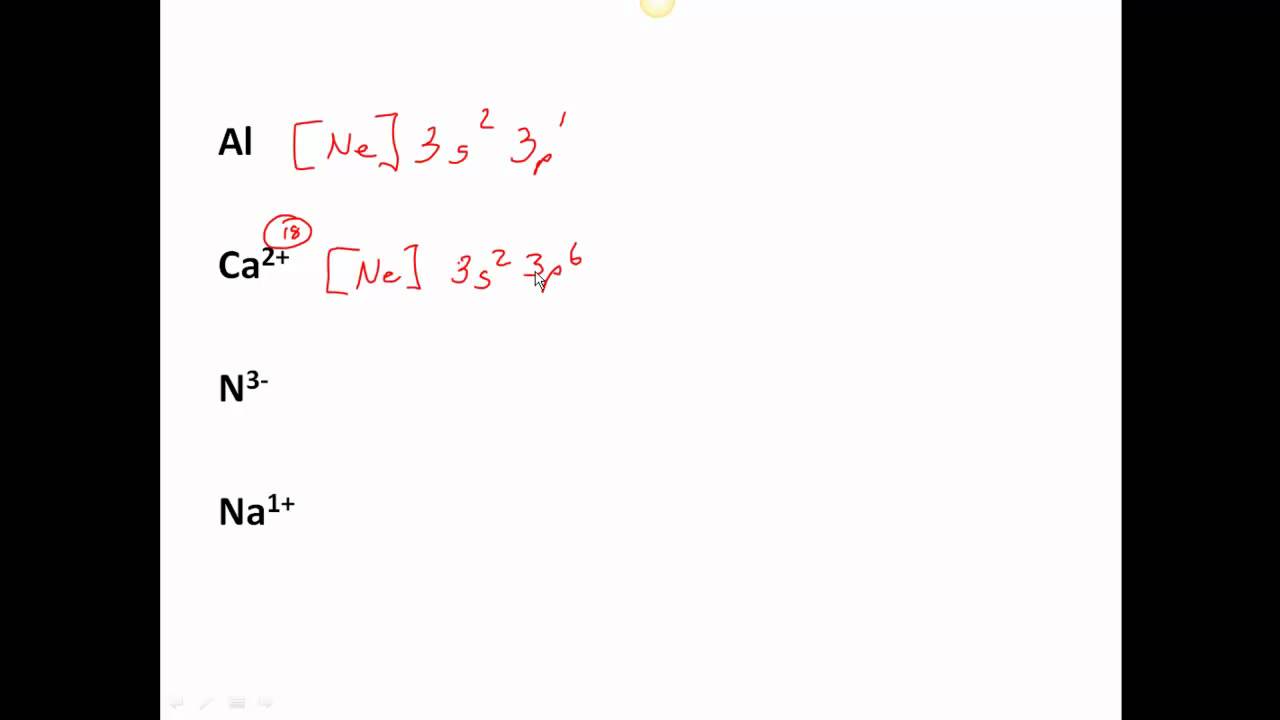
07 Abbreviated Electron Configurations YouTube
PROBLEM 3.1.13 3.1. 13. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse.". Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Answer.

The Electronic Configuration is the distribution of electrons of an
The last electron added is a 3p electron. Therefore, n = 3 and, for a p-type orbital, l = 1.. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons.

Orbital Diagram For Nitrogen N Nitrogen Electron Configuration My XXX
The nitride ion is N^ (-3) The original electron configuration for nitrogen is 1s^2 2s^2 2p^3 In order to fulfill the octet rule, the nitrogen atom would take on three additional electrons giving nitrogen a -3 charge. N^ (-3) 1s^2 2s^2 2p^6 I hope this was helpful. SMARTERTEACHER
/ColorPeriodicTableEC-58b5c7fa3df78cdcd8bbb56f.png)
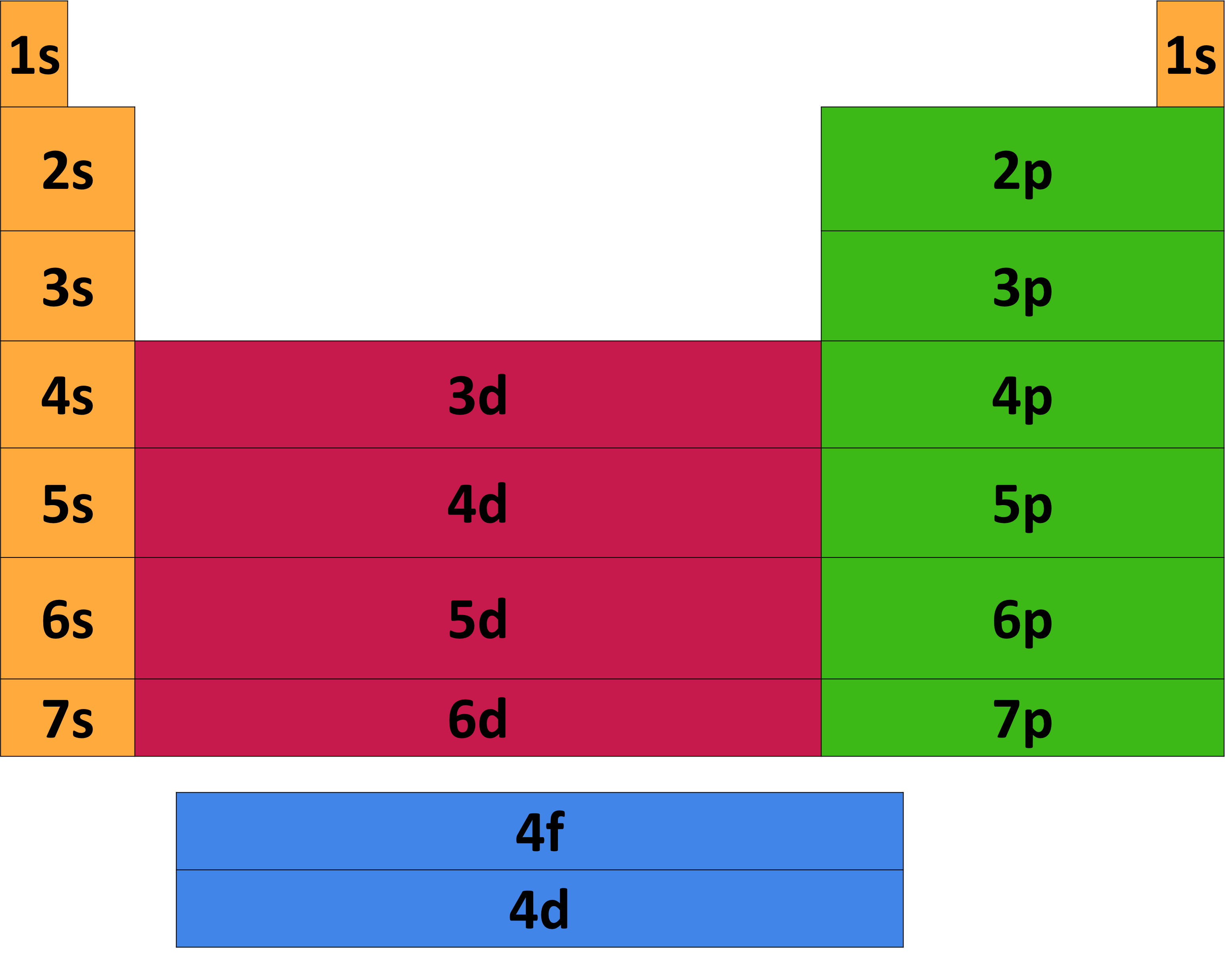
Download the Periodic Table With Electron Configurations
Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen Electron Configuration Notation The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom. This makes it easier to understand and predict how atoms will.
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Electron Configuration Chart
AboutTranscript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.

Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The
can be found. The three coordinates that come from Schrödinger's wave equations are the principal (n), angular (l), and magnetic (m) quantum numbers. These quantum numbers describe the size, shape, and orientation in space of the orbitals on an atom. The principal quantum number(n) describes the size of the orbital.

CC Electron configurations a must know hack
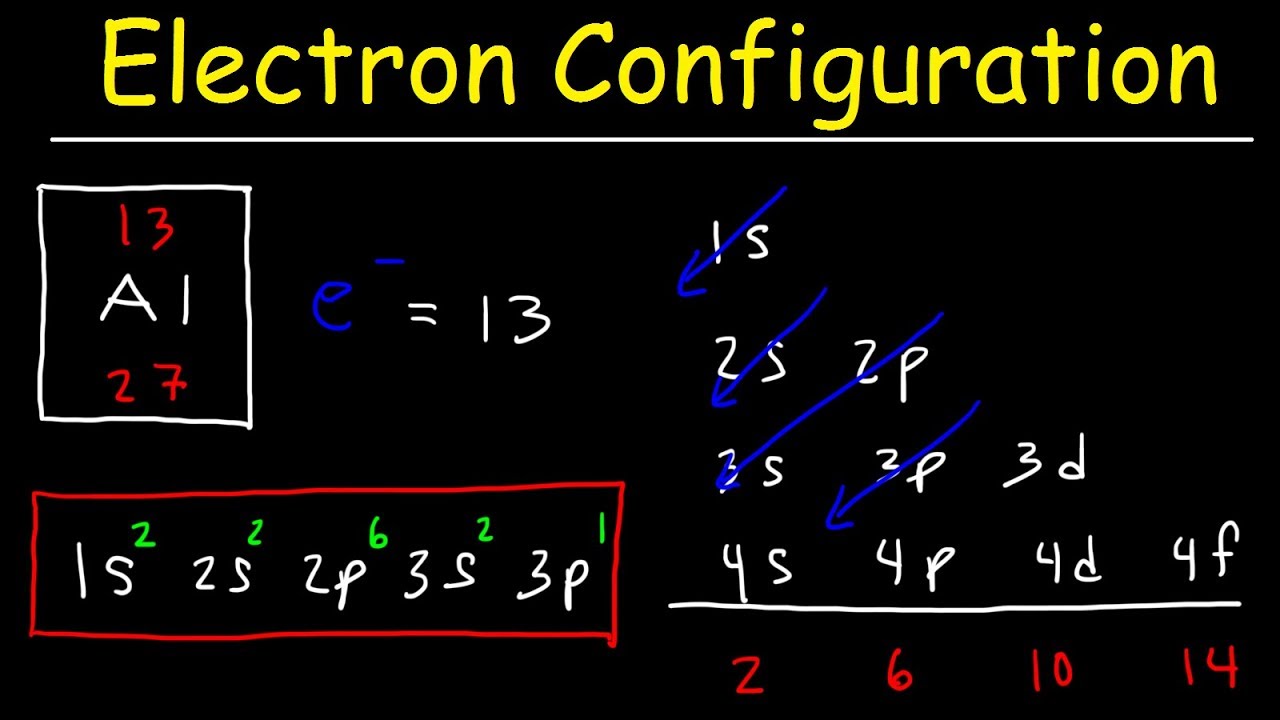
When writing the electron configuration for an atom, orbitals are filled in order of increasing atomic number. However, there are some exceptions to this rule. Example 3: 3 rd row elements. Following the pattern across a period from B (Z=5) to Ne (Z=10), the number of electrons increases and the subshells are filled.